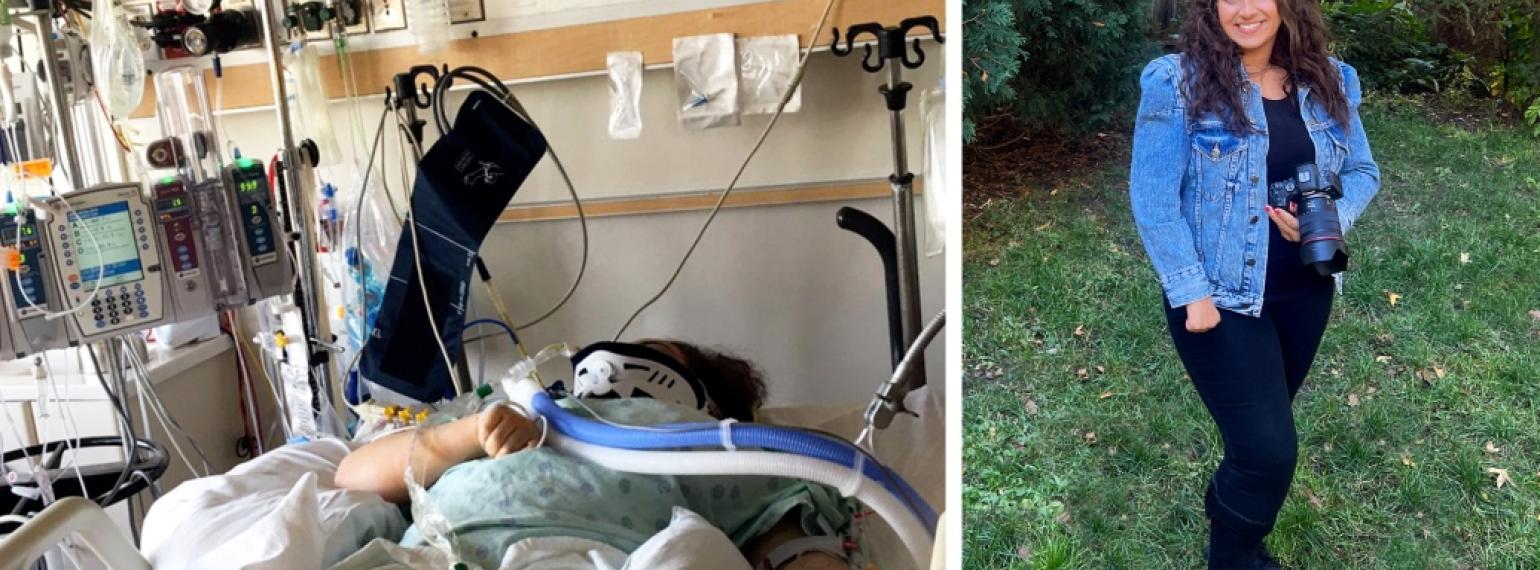
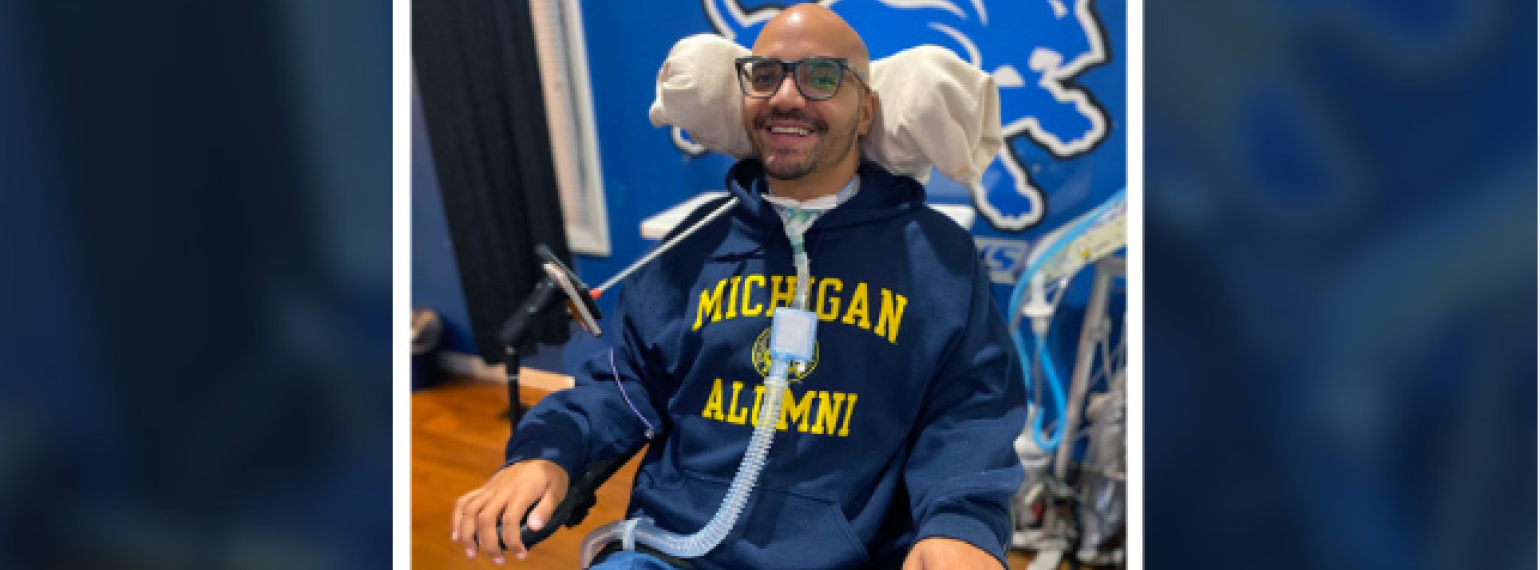
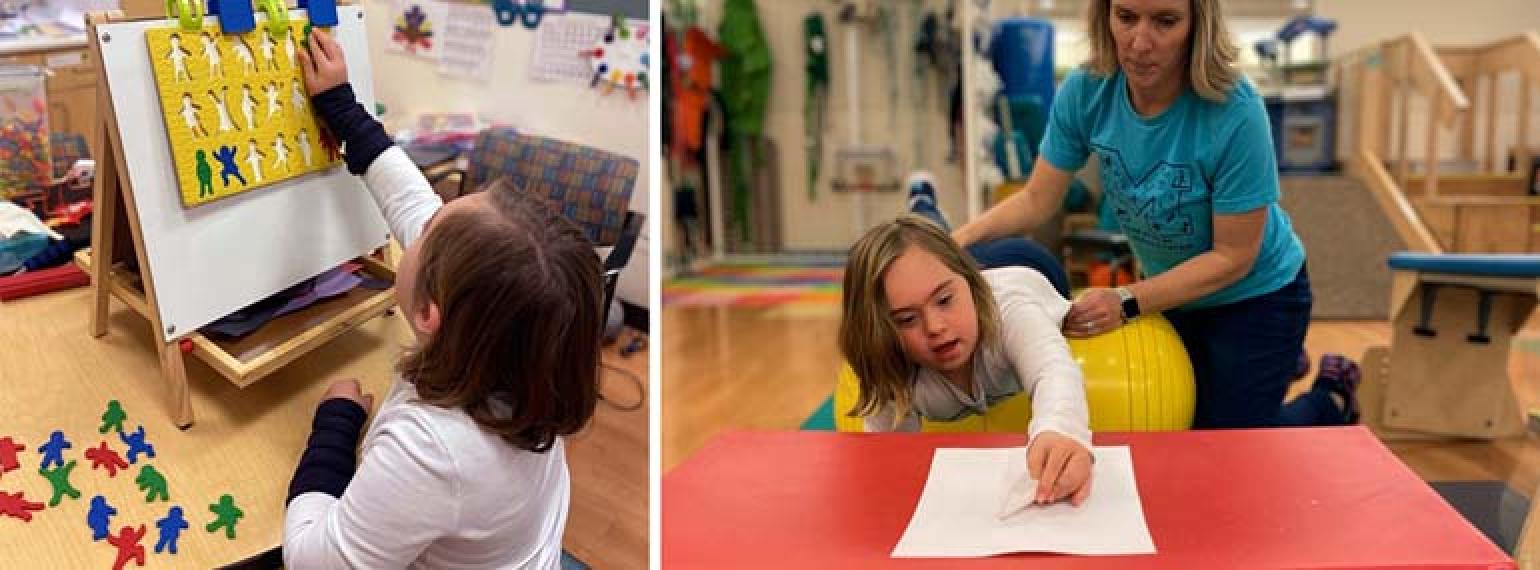Our goal is to help every individual maximize functional performance and achieve independence and community integration. Our methods involve an interdisciplinary team that works to increase function, decrease pain and disability, and maximize performance at work, in school, during recreation and in all other areas of life.
Our Mission
Maximize the health, function, and quality of life of individuals with acute and chronic illness and disability in the local and global community throughout their life span
Our Vision
Be the world leader in physical medicine and rehabilitation through excellence in innovative research, education, advocacy and interdisciplinary clinical practice
Our Values
Respect, Leadership, Collaboration, Sustainability, Innovation, Excellence, Advocacy, Inclusion
Anti-Racism Statement
Michigan Medicine unequivocally recognizes racism as a public health crisis, and we should be standing out as leaders against inequality. We are committed to creating fundamental change that leads to a culture of anti-racism, and a medical school and health system that are leaders in equity, justice and inclusiveness for people of all colors.
Visit the Anti-Racism Support and Tools section of the Office for Health Equity and Inclusion's website for more information. They have compiled a list of support resources to help individuals, teams and departments fight racism.